Moisture is involved in most building problems. The most serious tend to be structural damage due to wood decay, unhealthy fungal growth, corrosion, freeze-thaw, and damage to moisture sensitive interior finishes. Avoiding these problems requires an understanding of moisture, the nature of materials, and how it interacts with materials. This digest deals with these fundamentals.
Materials
It is common to classify materials in different ways. For example, organic materials are based on carbon and hydrogen molecules, whereas mineral-based materials are based on molecules with silicates and calcites. Another useful distinction is porous or non-porous materials; many materials are porous, and if the pores are microscopic (e.g., on the order of thousandths of an inch) they will have very large internal surface areas. For example, the interior surface area is about 0.2 m2/gram (50 ft2/ounce) for gypsum board, 20 m2/gram (5000 ft2/ounce) for cement paste and even more for wood or cellulose. Materials such as glass, steel and most plastics have essentially no porosity. Since these types of materials have no pores, they have no internal surface area and hence neither allow any significant water absorption nor moisture transmission.
The Water Molecule and its States
Water, like most materials, exists in liquid, solid, vapor and adsorbed states or phases. Unlike most materials, we experience water in all of its states during our daily lives. Iron and gasoline also exist in all of the states shown in Figure 1, but only at temperatures outside our normal range of experience. Of the states, the adsorbed state is the least understood, and will be described in more detail later.
Moisture, in all its states, is a molecule with two positively charged hydrogen atoms, and one negatively charged oxygen atom (H2O). The molecule is only about 0.3 nanometers in diameter: one billion laid end to end would be about one foot long.
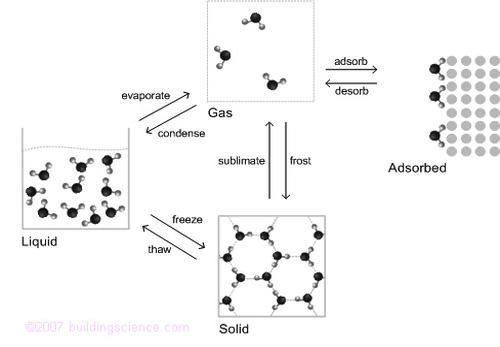
Figure 1: Moisture States and Phase Change Processes
Notice in Figure 2 that the centroid of the two positive charges is not coincident with the centroid of the two negative charges. This spatially-unbalanced distribution of charges means that H20 is a polar molecule that behaves like a tiny magnet, i.e., the hydrogen end is permanently positive and the oxygen end is permanently negative.
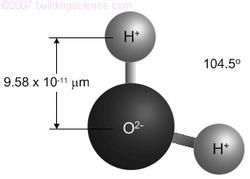
Figure 2: The Water (H20) Molecule
Water's Interaction with Materials
The polar water molecule "magnet" is attracted to many materials in both the vapor and liquid state. For example, water drops will cling to your skin and to the mirror after a shower. Liquid water is actually sucked into the very small tubes (termed capillaries) present in porous materials: the smaller the tube the greater this capillary suction. The suction (or wicking) of interconnected capillaries explains how water is drawn up into celery, into brick, and to the top of tall trees. A few materials, like silicone, oils, and some plastics repulse water, and this repulsion causes water to bead up (e.g., rain on oil-soaked concrete, or water on wax paper).
The manner in which water molecules interact with other water molecules is also due to their polar nature. The hydrogen of one molecule attracts the oxygen of another and causes the water to group together. Hence, liquid water tends to exist in large clusters. As the temperature increases, the clusters gain more energy and break up into smaller clusters. When liquid water is evaporated, the molecules gain so much energy that they act individually as lone vapor molecules. The difference in size between the liquid water molecule clumps and the lone vapor molecule helps explain how materials such as Gore-Tex™ and Tyvek™ can simultaneously be watertight and highly vapor permeable.
Moisture Storage and Material Response
Many surfaces in contact with water vapor molecules have the tendency to capture and hold water molecules because of the polar nature of the water molecule; this process is called adsorption. These materials are called hydrophilic, whereas materials that repel water are called hydrophobic. Most building materials are hydrophilic. As water vapor molecules in the air adsorb to the internal surfaces of these materials, the materials' water content increases significantly. Such materials are described as hygroscopic. Materials such as glass, plastic, and steel do not have internal pores and therefore are not hygroscopic — they do not pick up moisture from water vapor in the air. (Desiccants are a special type of hygroscopic material. They can absorb a very large amount of moisture, typically several times their dry weight at high relative humidities).
When a material has adsorbed all the moisture it can, further moisture will be stored in the pores and cracks within the material by capillary suction, or by absorption. For example, wood will adsorb vapor from the air up to approximately 25 or 30% moisture content at 98% relative humidity, but fully capillary saturated wood wetted by liquid water may hold two to four times this amount of moisture. Once a material is capillary saturated it will generally not be able to store any more moisture. When this moisture content is exceeded, a material is called over-saturated, and no more water can be wicked into the material and drainage mechanisms, if available, will begin to remove the excess moisture.
In summary, liquid water is absorbed into capillary pores, and significant amounts of water vapour can be adsorbed to the surface of pore walls.
Moisture Storage Regimes
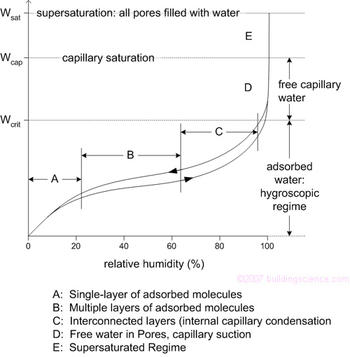
The figure below shows the three different regimes of moisture storage: the sorption or hygroscopic regime (Regions A-C), the capillary regime (D) and the over-saturated regime (E). It is very important to recognize that the moisture content varies primarily with relative humidity, not with absolute humidity.
In the hygroscopic regime, water vapor adsorbs to the pore walls. As RH increases, more layers adhere, although the first few layers are more strongly attached (Regions A and B). In Region C, the layers grow to such a size that they begin to interact and interconnect and the surface tension of water causes meniscuses to form within the smallest pores. At the highest relative humidities, all but the largest pores are filled with water. The capillary regime (Region D) is somewhat arbitrarily designated as that part of the moisture storage function above the critical moisture content. Physically, it is presumed that a continuous liquid phase forms. Finally, in the supersaturated state, the RH is always 100% and no more water will wick into a material – external forces (like gravity and air) must force it in.
Practical Implications
Materials such a wood, mortar, gypsum, and concrete begin life with all of their pores filled with water. As the material dries, the water content in the pores drops and adsorbed water will try to leave the surface of the pores. However, the strength of the bond between water molecules that make up the adsorbed layers results in tension forces as drying progresses. It is these internal tension forces that cause drying shrinkage stresses and the consequent cracking in wood, clay, concrete, and plaster.
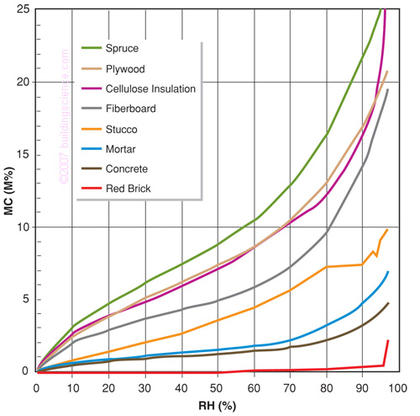
Figure 4: Sorption Isotherms for Many Building Materials
Conversely, brick begins life in a high temperature oven: completely dry. When water vapor subsequently enters the pores, compressive forces are developed as water molecules force themselves close to the brick material and other adsorbed water molecules. This internal compressive force causes the initial expansion of brick, and the wetting expansion of other porous materials like clay and wood.
Hence, wetting and drying due to adsorption will cause expansion and contraction in many materials. This explains why wooden doors tend to be tight in the humid summer and loose-fitting in the dry winter. Differential moisture-related expansion and contraction also explains cupping in woods and slab curl in concrete.
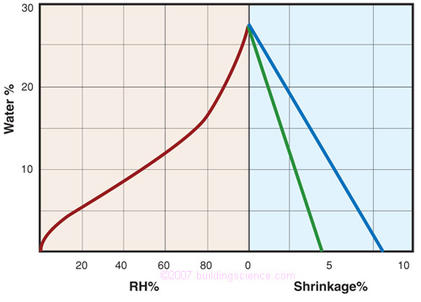
Figure 5: The moisture content versus RH and shrinkage versus MC of a typical wood
In the upper portion of the hygroscopic regime (typically once the relative humidity exceeds about 80%), the adsorbed water vapor is attached so loosely that it may be available for fungal growth and corrosion. However, for dangerous levels of corrosion and mould growth, much higher humidities and even liquid water may be needed.
Time, temperature and relative humidity are the most important environmental variables affecting durability. (The moisture content can be related to the relative humidity by the sorption isotherm). Fungal growth can begin on most surfaces when the stored moisture results in a local relative humidity of over about 80% after many months. Corrosion and decay require higher levels of humidity (well over 90%) and temperatures over 60 F (15 C) for months, to proceed at dangerous rates. It is for these reasons that the RH around a material should be controlled, NOT the moisture content. Freeze-thaw damage and dissolution (of, for example, gypsum) require the material to be at or near capillary saturation (100%RH).
To dry out a material previously wetted, one must reduce the relative humidity within the materials as quickly as possible. It should be clear that drainage is not sufficient for this purpose since it will leave a large amount of saturated (100%RH) material. Capillary and adsorbed moisture can only be dried by evaporation followed by diffusion.
Summary and Conclusions
Water is a unique and fascinating molecule. Understanding how and why moisture interacts with materials is critical to the design, construction, and operation of healthy, durable, and energy efficient buildings.